Spiral Tracing Assessment Workflow
Spiral Tracing [Software as a medical device]
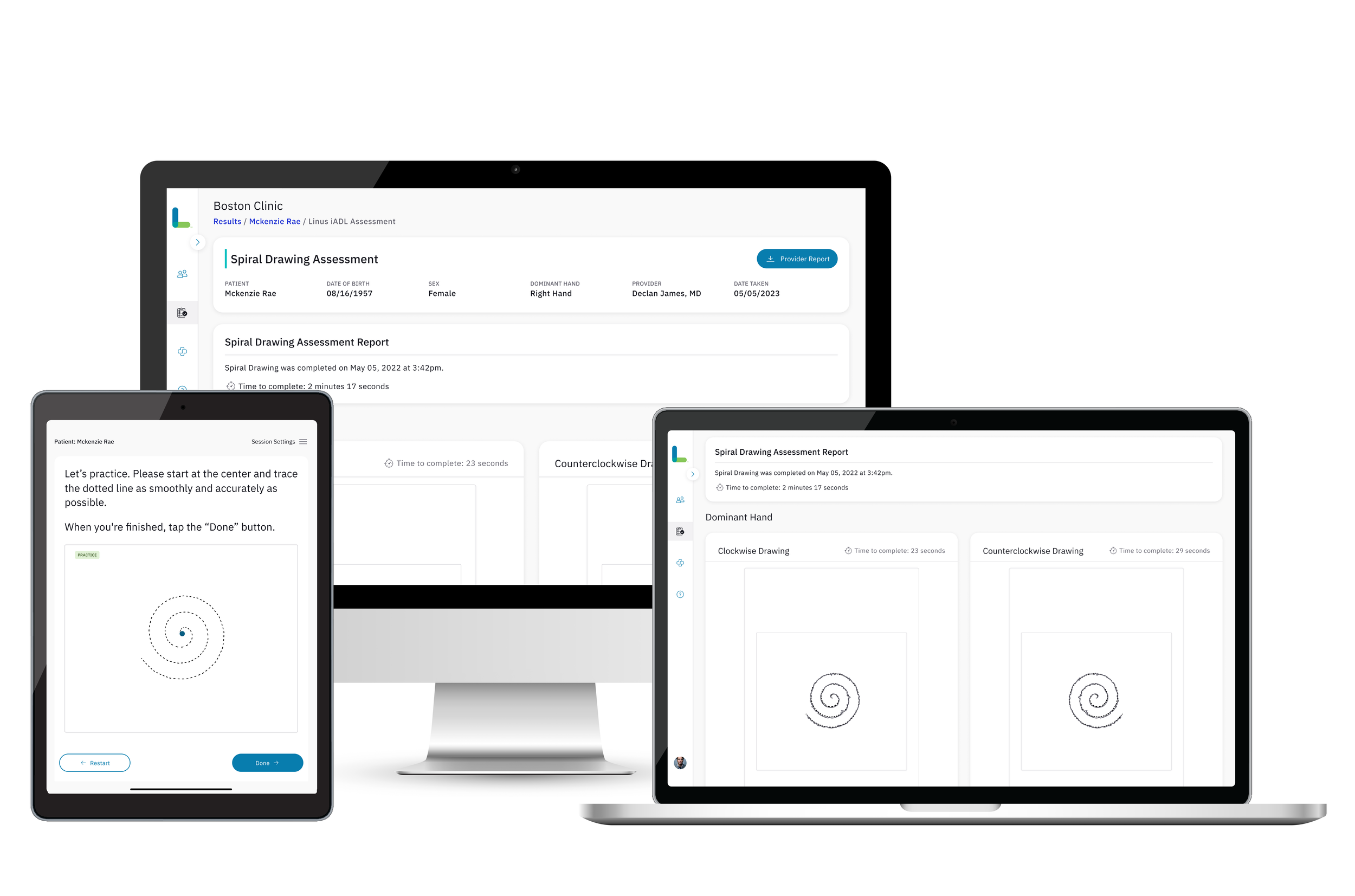
The Spiral Tracing Assessment is a neuropsychological assessment tool used to evaluate fine motor skills, visual-motor integration, and cognitive functioning. It is sometimes used in clinical settings, particularly in assessments of neurological conditions, cognitive impairments, or developmental disorders.
Given the Linus DCR/CEE's capability to detect issues such as Tremors and Parkinsonism concerns, we proceeded to digitize the Spiral Tracing Assessment to enhance the identification and quantification of tremors, including their frequency and amplitude. This digitization facilitates automated assessment, allowing our data analytics team's engine to trigger additional quantitative measures such as speed, path length and precision in the tracing process.
Exploring innovative Alzheimer's disease (AD) biomarkers, we consider motor functional outcome measures as potential candidates, given that motor impairments may precede cognitive dysfunction. This digitized version enables the evaluation of fine motor function in Mild Cognitive Impairment (MCI) and AD, comparing it to cognitively normal individuals. Data suggests that individuals with AD exhibit less precision in tracing the spiral, despite maintaining adequate movement speed and steadiness.
Instant Results
Shortly after designing our initial assessment in 2021, we recognized that Medical Assistants (MAs) prefer instant access to results on the testing device (an iPad provided by Linus). We successfully implemented this feature within one quarter for our customers. Now, it is a standard practice for every assessment to provide scores immediately on the Admin App, accompanied by access to the PDF, which is automatically uploaded to the Electronic Health Record (EHR). This ensures MAs can efficiently triage next steps.
Personas
Older Adults/Patients (aged 55+): This user would be an older adult (him/her/them), who may live independently in the community or in an assistive facility and may visit clinics for regular or acute check-ups. In the Linus platform Patients will have the ability to take assessments and view patient facing reports.
Practice Managers: The person who manages the operational aspects of a clinic or practice. In the Linus platform they will have a Clinical Administrator role and have the ability to add and deactivate users in the system.
Clinicians (ex: MD, DO, NP, PA, RN, MA): This can be any healthcare provider or other qualified healthcare professional. In the Linus platform they will have the Clinician role which will give them the ability to add patients, administer assessments and view reports and recommendations.
Primary Site Contacts, Manager Role: These are contacts at our clinical sites who may have responsibility across multiple sites and may want to view patients across many clinic locations within a parent organization. In the Linus platform, Managers have the ability to view the web portal for multiple clinic locations, but they cannot administer assessments.
Linus Admin: A Linus Health employee in the Customer Support department who configures the software for customers and access the system data to help troubleshoot and guide customers through their use of the system.
Researcher: An academic, pharmaceutical or clinical researcher interested in capturing and analyzing tremor or cognitive assessment data.
Personas
We crafted Fictional Characters based on our UXR research, representing diverse user types who may engage with our product or service features. Developing personas is instrumental in comprehending our users' needs, experiences, behaviors, and goals. It enables us to step into the users' shoes, acknowledging their varied needs and expectations. Personas simplify the design process, guide our ideation, and contribute to creating a positive user experience for the target user group.
Regulatory Documentation Pages from the Spiral User Design Definition Document (UDDD), Use Specification, Risk Analysis, User-Interface Requirements (in compliance with regulatory agency expectations
Regulatory Documentation
Each assessment classified as a medical device by my team necessitates specific documentation. We create User Definition Design Documents tailored for Product Designers and Software Developers involved in the design's implementation, verification, and maintenance. This document references incoming requirements containing the design, describes subsystem design details, and defines the deliverables. It excludes descriptions of hardware-user interactions.
Within this document, a section is dedicated to a UI Evaluation Plan, outlining usability validation for upcoming projects. For assessments, we typically conduct summative user studies post-implementation by engineering and before market launch. We validate safe and effective use through user research and show its effectiveness-or success or failure rate. During summative testing, we focus on use errors and usability risks, as our patient-facing assessments' design significantly influences results, impacting the understanding of the disease process. Consequently, the design and process of medical devices play a crucial role in Linus's regulatory infrastructure.
Go back to Assessment List | Go back to Assessment Library Overview